This PPT contains,
Introduction
Mechanisms of RNA processing
Processing of pre-mRNA
5’ capping
Polyadenylation
Types of polyadenylation
Intron splicing
Exon and intron Definition
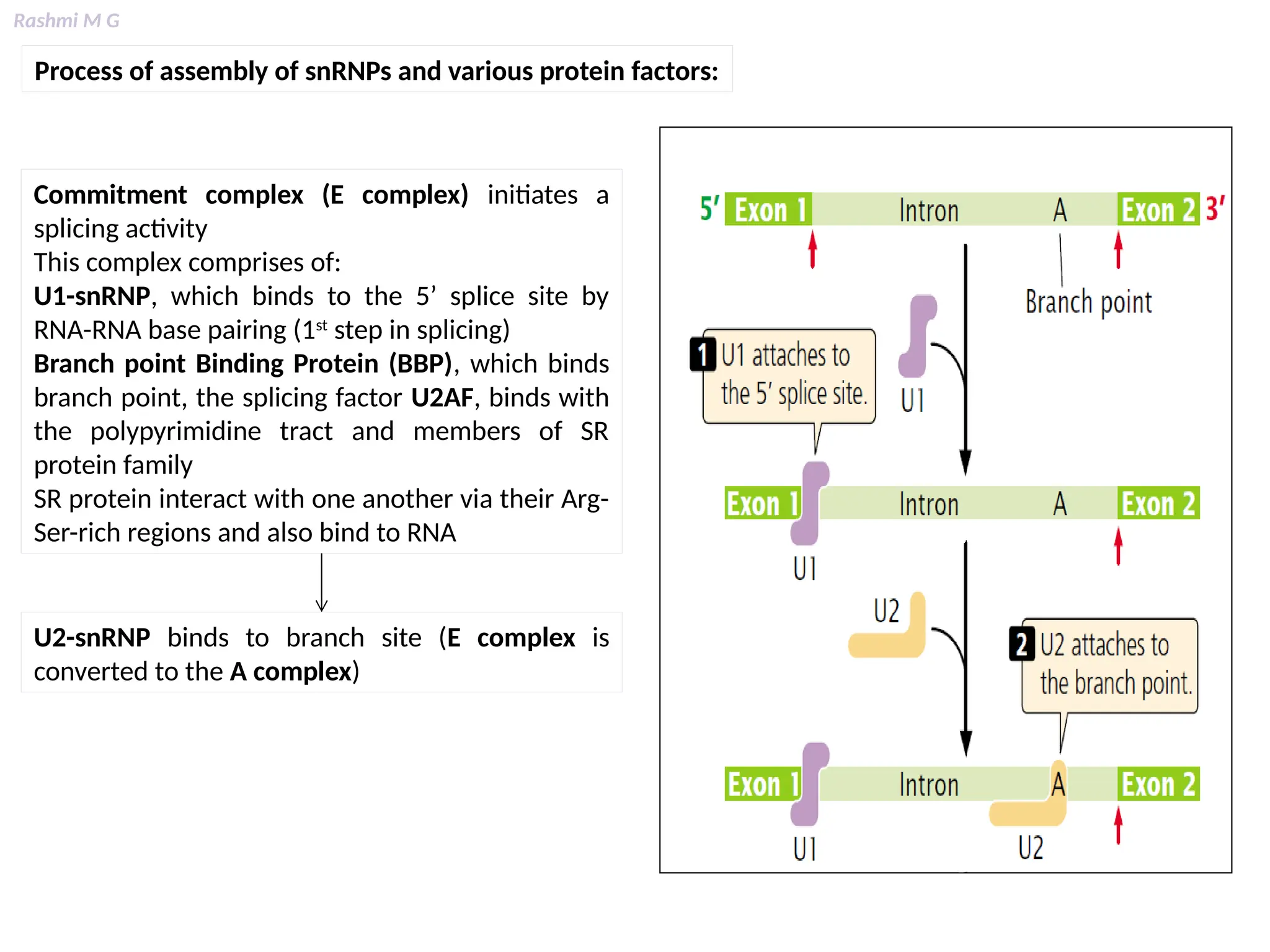
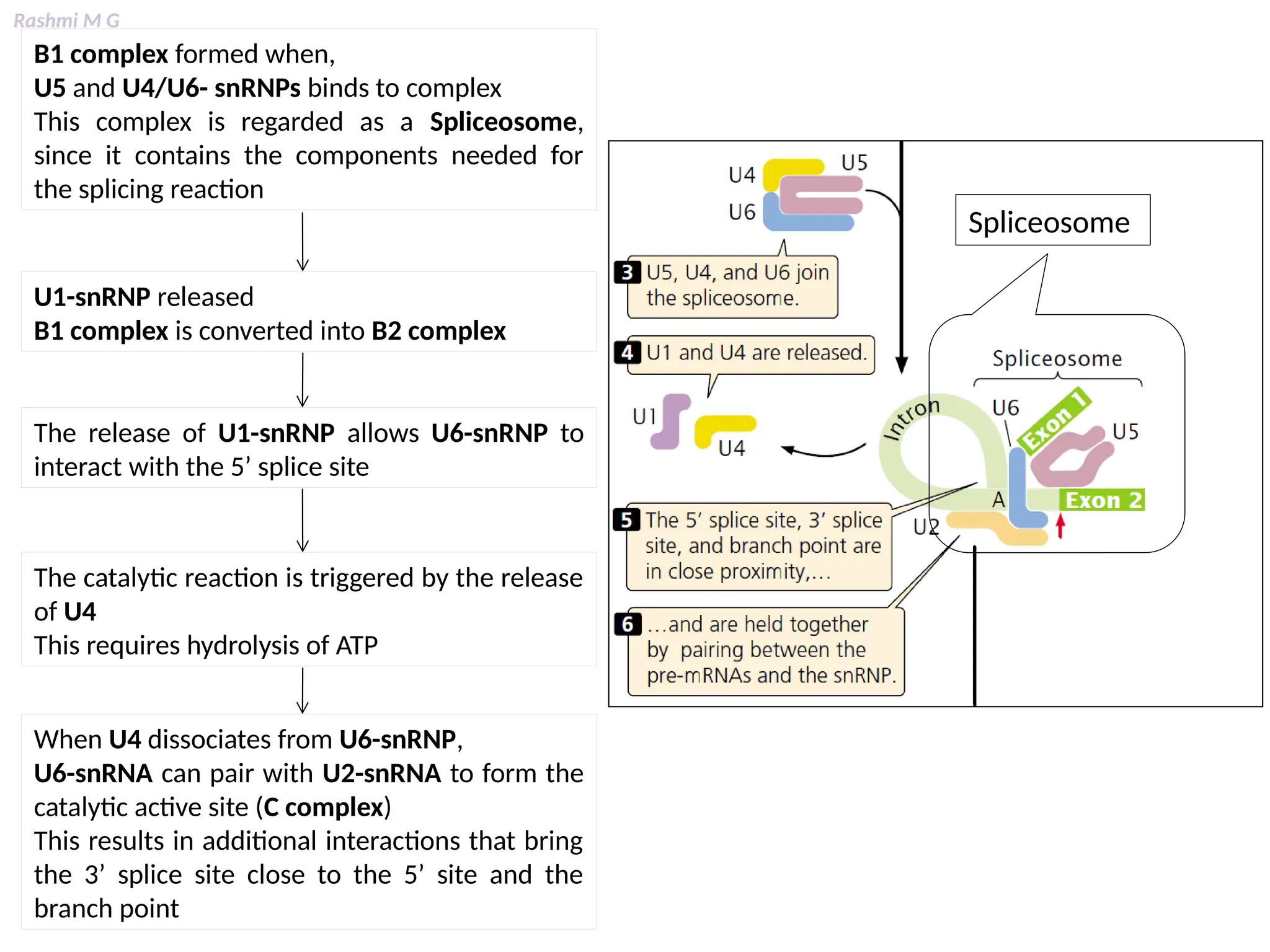
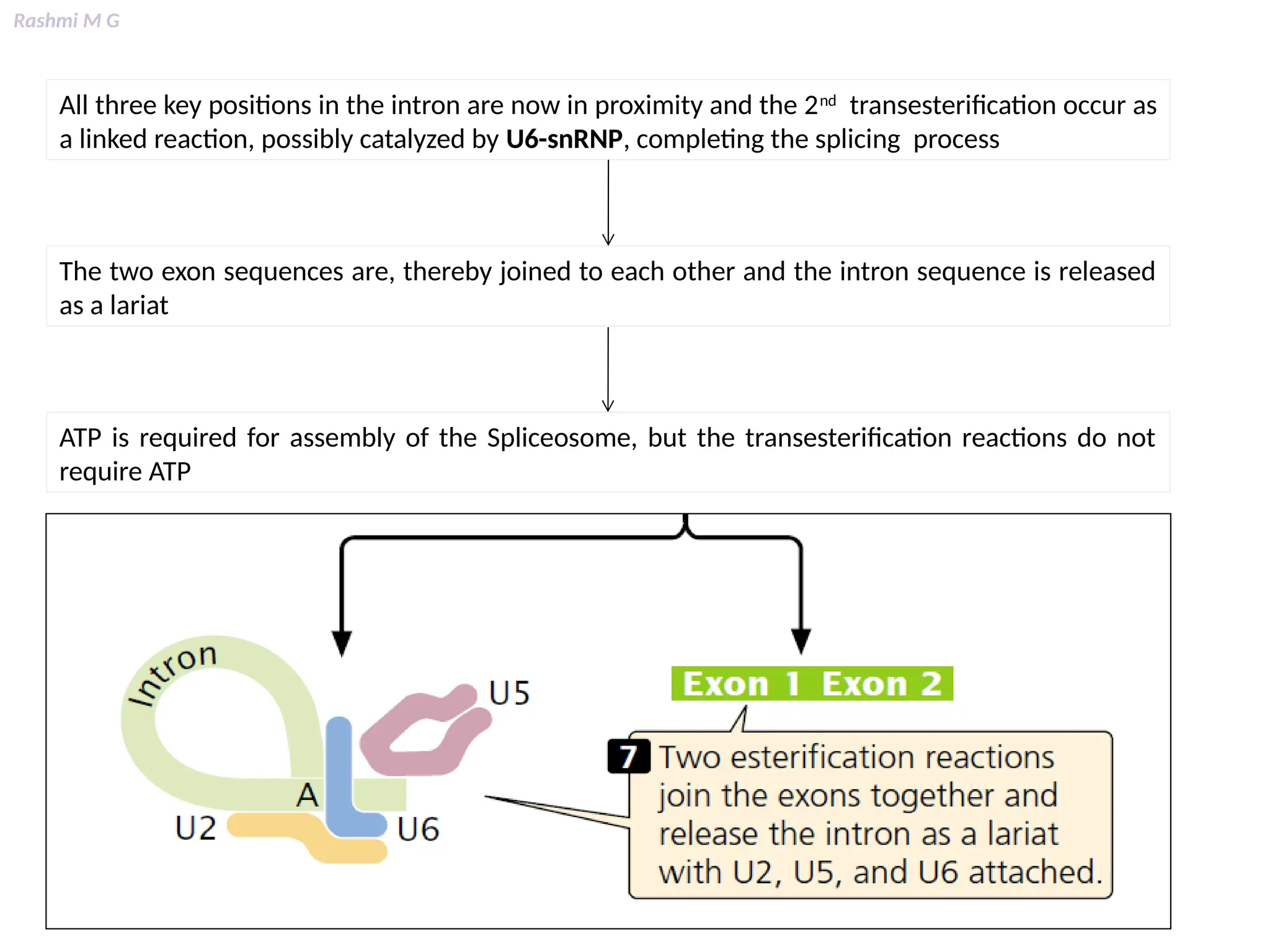
Splicing apparatus
Alternative splicing
Trans splicing
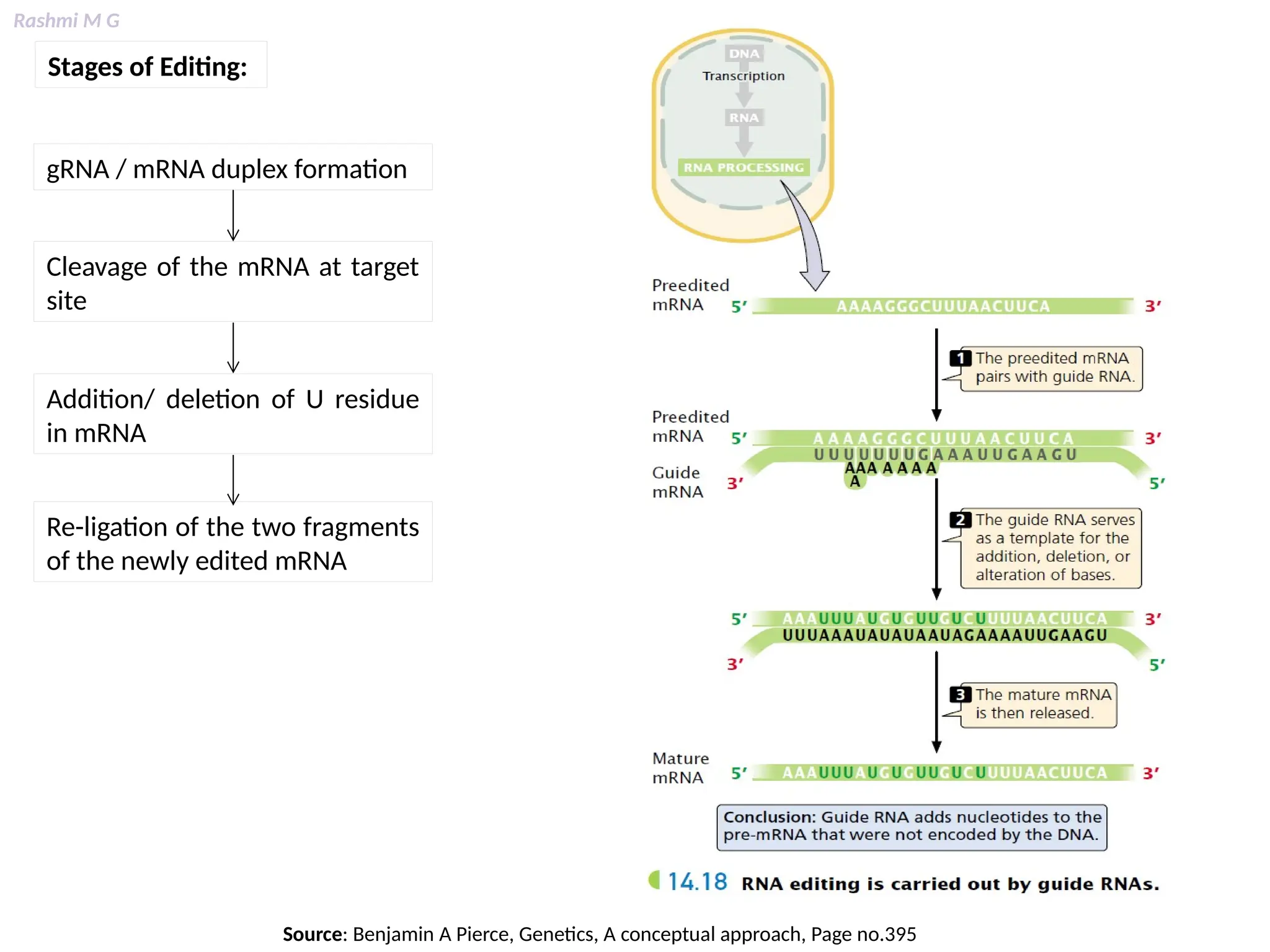
RNA editing
Processing of pre-rRNA
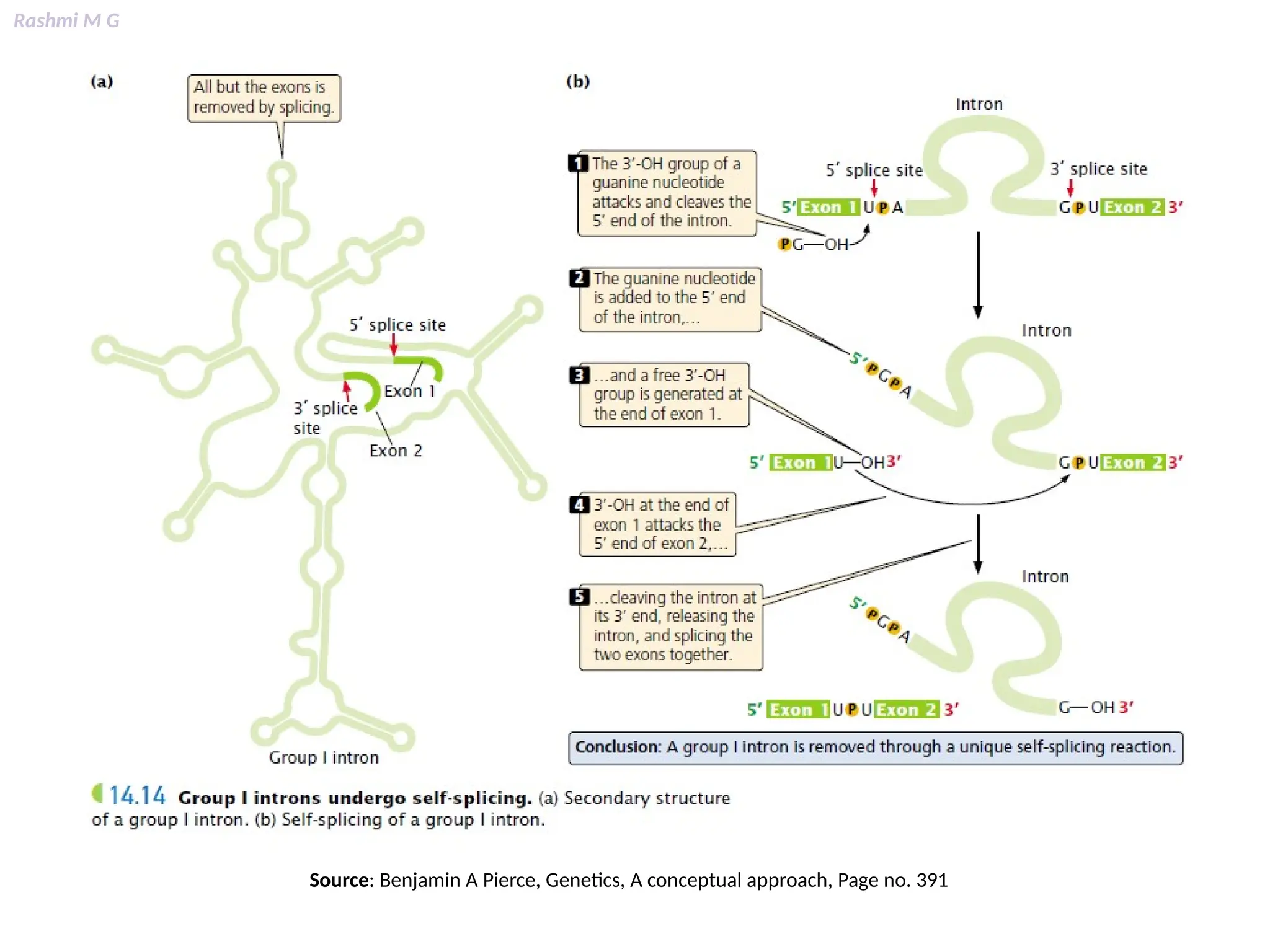
Group I and group II introns
Processing of pre-tRNA
mRNA degradation
mRNA surveillance